
It is found naturally as a translucent emerald-green crystal formed in thin sheets near the boundaries of idocrase or chlorite crystals. The mineral form of Ni(OH) 2, theophrastite, was first identified in the Vermion region of northern Greece, in 1980. In addition to the α and β polymorphs, several γ nickel hydroxides have been described, distinguished by crystal structures with much larger inter-sheet distances. In the presence of water, the α polymorph typically recrystallizes to the β form. The β form adopts a hexagonal close-packed structure of Ni 2+ and OH − ions. The α structure consists of Ni(OH) 2 layers with intercalated anions or water. Nickel(II) hydroxide has two well-characterized polymorphs, α and β. It is electroactive, being converted to the Ni(III) oxy-hydroxide, leading to widespread applications in rechargeable batteries.
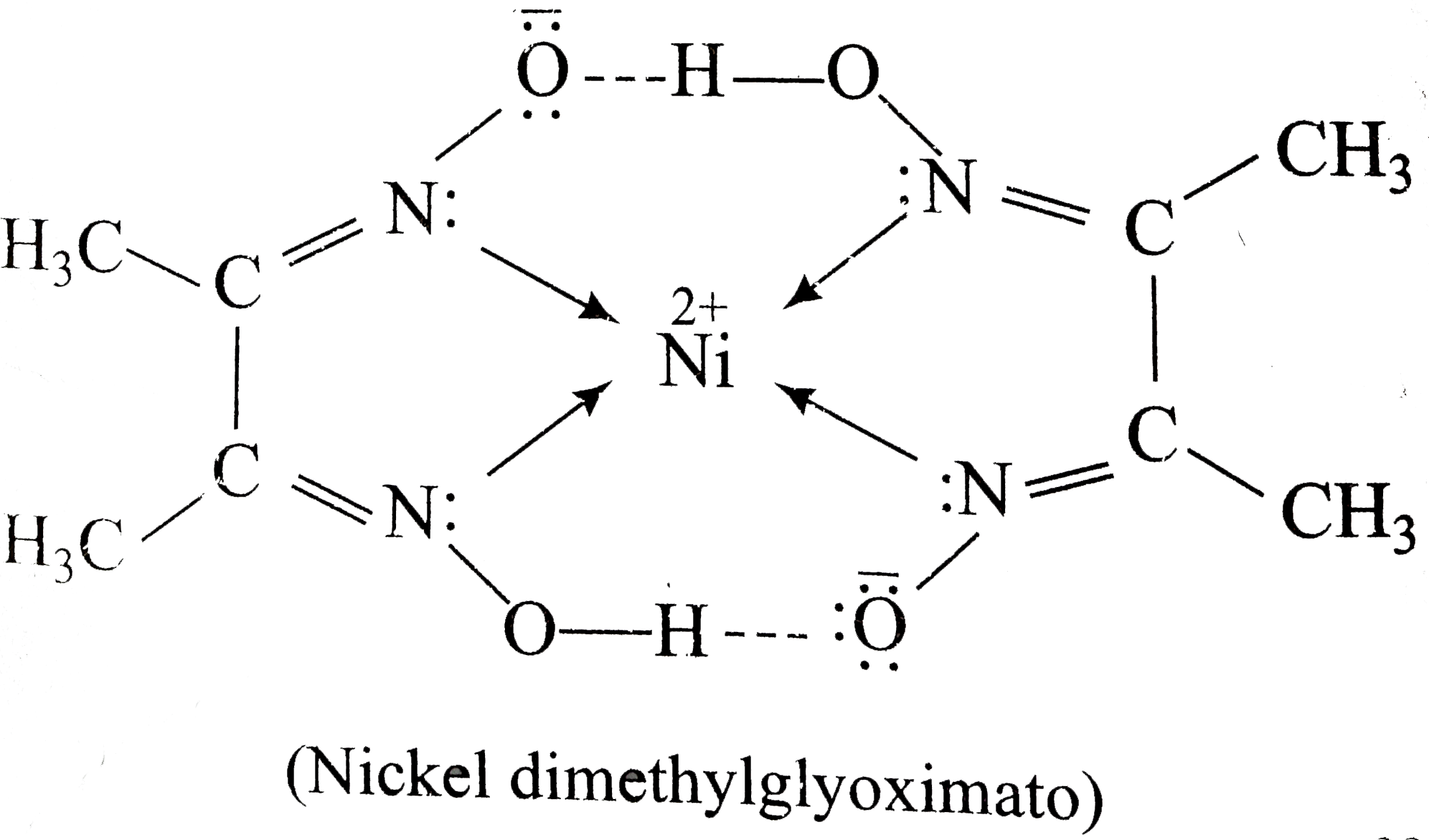
It is an apple-green solid that dissolves with decomposition in ammonia and amines and is attacked by acids. Nickel(II) hydroxide is the inorganic compound with the formula Ni(OH) 2. Its coordination complexes are of theoretical interest as models for enzymes and as. DmgH 2 is used in the analysis of palladium or nickel.

This colourless solid is the dioxime derivative of the diketone butane-2,3-dione (also known as diacetyl). Its abbreviation is dmgH 2 for neutral form, and dmgH for anionic form, where H stands for hydrogen. Relevant Equations: I believe you take the mass of the DMG-Ni complex sample and multiply that by the mass ratio of Ni2+ over the FW of DMG. We did a gravimetric analysis lab and I have masses for the DMG-Ni complexes that we recovered but I need to find the percent nickel in each sample. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. ››More information on molar mass and molecular weight. High alkalinity in the sample solution causes a leaching of Ni(DMG)2. The suitable operational pH range for the nickel infrared sensor is between 6 - 8. The detection of Ni2+ is based on the appearance of a unique infrared absorption peak at 1572 cm(-1) that corresponds to the C=N stretching mode in the nickel dimethylglyoximate, Ni(DMG)2, complex. Finding molar mass starts with units of grams per mole (g/mol).

For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. Please let us know how we can improve this web app.The test tube in the middle contains a precipitate of nickel(II) hydroxide Related: Molecular weights of amino acids Weights of atoms and isotopes are from NIST article. Molar mass ( molar weight) is the mass of one mole of a substance and is expressed in g/mol.

(1 u is equal to 1/12 the mass of one atom of carbon-12)


 0 kommentar(er)
0 kommentar(er)
